QbD und DoE Statistische Werkzeuge: Hypothesentests und Regression Screening Designs: Identifizieren wichtiger Faktoren Response-Surface Designs: Definieren von Design-Spaces Bestätigung von Design-Spaces
Design Expert - Quality by Design
Product information "Design Expert - Quality by Design"
Course: Quality by Design (QbD) – Statistical Experimental Design for Pharmaceutical Processes
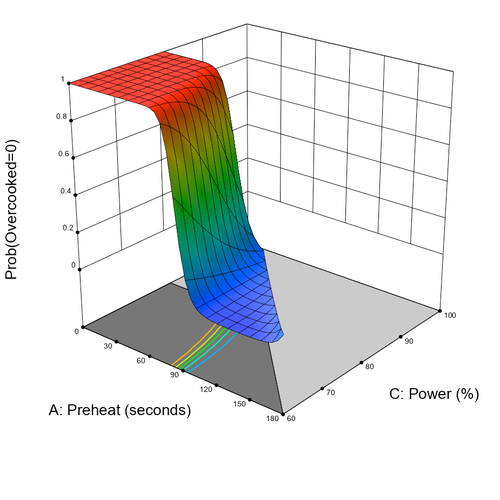
Quality by Design (QbD) is the FDA-recommended method for developing and optimizing pharmaceutical products and processes. The central idea is to define factor spaces within which consistent product quality is ensured. Once a design space is approved by regulatory authorities, the process can be adjusted within these limits without requiring re-approval.
This methodology provides new flexibility in the production of pharmaceutical products and medical devices, reducing regulatory hurdles.
What You Will Learn in This Course:
📌 Fundamentals of Quality by Design (QbD) & Design of Experiments (DoE)
📌 Statistical Tools: Hypothesis Testing & Regression Analysis
📌 Screening Designs: Efficiently Identifying Key Factors
📌 Response Surface Designs: Defining and Optimizing Design Spaces
📌 Validation of Design Spaces for Regulatory Approval
📌 Practical Implementation with Design-Expert® Software
The course will follow a complete QbD cycle using FDA examples and statistically model it with Design-Expert® software.
Requirements:
📌 Basic knowledge of Design-Expert® is beneficial.
📌 Knowledge of regression analysis and hypothesis testing is helpful but not required.
🕒 Course Duration: 3 Days
🔹 Sign up now and optimize your pharmaceutical development!
Details
Keine